CONTENTS
These are some of the scientific papers I consulted and took particular note of as I prepared an update on the ‘soil-acid-rain story’. For some cited below, the full paper is available online, others not so, but as a retired academic I had access to the full papers via my university. To be continued! -dp Feb 15, 2022.
– SOME HISTORY (to 2000)
– Effects on Fish
– SULFUR DIOXIDE EMISSIONS
– SOME HISTORY post- 2000
– SOME RELATED PUBLICATIONS BY K. KEYS & COLLEAGUES
– SOME RELATED RECENT PUBLICATIONS BY S.STERLING & COLLEAGUES
– DIVERSE EFFECTS OF LOSS OF CALCIUM
– Some Watershed-level effects of forest management
SOME HISTORY (to 2000)
| Early chronology. From 1. Acid Rain. O. Bricker and K. Rice. 1993 Annu. Rev. Earrh Planet. Sci. 1993. 21:151-74 2.Acid Rain. Alan Nixon, Thomas Curran. 1979. Last Revision in 1998. Science and Technology Division of the Parliamentary Research Branch Mid 1600s Latter 1800s 20th Century “When the acidification of lakes was first described in Ontario in the early 1950s, the phenomenon was considered to be simply a local problem resulting from the lakes’ proximity to nickel smelting operations in and around Sudbury.”[2] “From Smith’s time until the mid-20th century there was no focused systematic program of research on acid deposition. The connection between acid deposition and damage to ecological systems was once again demonstrated a century after Smith’s pioneering work by an American limnologist, Eville Gorham, and his colleagues in a series of papers pub lished between 1955 and 1965 (e.g. Gorham 1961, also see Gorham 1976). Their work, like that of Smith, did not receive much attention at that time.’ [1] Late 1960s into 1970s “About the same time that Oden was documenting the acid rain problem in Scandinavia and Europe, researchers were discovering similar problems in the eastern U.S., particularly in the northeastern States…”[1] 1970s & 80s: Watershed Level Processes “The mineralogy of the watershed substrate is a good predictor of the sensitivity of waters in the system to chronic acidification by acid depo sition (Puckett & Bricker 1992, Bricker & Rice 1989, Norton 1980). Waters in watersheds developed on carbonate rocks (e.g. limestones, dolomites, marbles) or reactive silicate rocks (e.g. dunites, serpentinites, gabbros, amphibolites) are not likely to become acidified because the minerals comprising these rocks rapidly react with H+. Waters in watersheds developed on silicious clastic rocks (e.g. sandstones, quartzites, siltstones, shales) or sialic crystalline rocks (e.g. granites, gneisses, schists, phyllites) are at risk of acidi cation because the minerals comprising these rocks do not react, or only react slowly, with H+. Stream baseflow chemistry strongly rflects the mineralogy of the watershed and is a good predictor of the susceptibility of a system to chronic acidi cation by acid deposition (Lynch & Dise 1985, Bricker & Rice 1989).” “During storms the chemistry of stream ow may change drastically from its base flow composition (Wigington et al 1990, Kennedy et al 1986). Streams that are well buffered under base flow conditions may experience a precipitous drop in pH and alkalinity at the peak of storm discharge or snowmelt runoff. Acute depressions of pH and alkalinity associated with runo events may last for hours to weeks and adversely affect the stream biota (Baker ct al 1990). Storm-induced changes in stream chemistry appear to be related to diffrences in the paths along which water flows through the system (Wheater et al 1990, Vogt et al 1990)…” [1] Conclusion in 1993 about effects on aquatic ecosystems & forests [1] “The effcts of acid deposition on aquatic ecosystems have been well documented and are understood at the large scale. Although many details at the fine scale have yet to be elucidated, there is no longer any doubt that acid deposition can have devastating effects on aquatic communities in poorly-buffered waters that are susceptible to both episodic and chronic acidification.” “Forest decline in a number of countries in Europe and North America has been attributed to acid deposition, although the evidence is not as strong as it is with respect to aquatic ecosystems. In Cechoslovakia, much of the forest in the Erzgebirge region has died and the remainder shows severe damage. In Germany, the Black Forest and forests in parts of Bavaria have undergone extensive dieback. In North America, forest damage appears to be confined ned largely to high-elevation red spruce forests in the eastern and northeaste parts of the continent. The details of the extent of forest damage directly caused by acid deposition relative to gaseous air pollutants (e.g. 03, S02) is not clear; however all of these pollutants largely originate directly or indirectly from common sources.” |
Acid rain: a serious regional environmental problem
G E Likens, F H Bormann. 1974 Science 184.4142.1176
Abstract: “At present, acid rain or snow is falling on most of the northeastern United States. The annual acidity value averages about pH 4, but values between pH 2.1 and 5 have been recorded for individual storms. The acidity of precipitation in this region apparently increased about 20 years ago, and the increase may have been associated with the augmented use of natural gas and with the installation of particle-removal devices in tall smokestacks. Only some of the ecological and economic effects of this widespread introduction of strong acids into natural systems are known at present, but clearly they must be considered in proposals for new energy sources and in the development of air quality emission standards.”
Freshwater acidification from atmospheric deposition of sulfuric acid: A conceptual model
James N. Galloway et al. 1983. Environ. Sci. Technol. 1983, 17, 11, 541A–545A “We have presented a simple conceptual model that we feel describes the dynamic response of surface water chemistry as a function of rates of acid deposition (notably H2SO4) and a few key soil processes. Work continues on the use of temporal water chemistry data as a means of qualitatively verifying the concepts underlying these seven stages of acidification….The authors wrote at the time that “Because sulfur deposition has not yet begun to decrease significantly, if at all, the pertinent characteristics of Stages 1-4 only are listed below
• Stage 1: Systems are not receiving acid deposition.
• Stage 2: Systems are receiving acid deposition, soil is unsaturated with S04, and the BC [Base Cations] concentrations are increasing.
• Stage 3: Systems are receiving acid deposition, soil is saturated with S04, alkalinity is decreasing, and the BC concentrations are decreasing.
• Stage 4: Systems are receiving acid deposition, soil is saturated with sulfur, alkalinity is constant but lower than initially, and the BC concentrations are constant.
From the text (i.e. predicted)
• Stage 5 Supersaturated SAC This stage begins with decreases in the deposition of S04 (and H+) resulting from decreases in sulfur emissions. Concurrently, the higher pH shifts the adsorption reactions, and both the terrestrial and aquatic systems (soil and sediment) scavenge cations, causing a decrease in BCs…
• Stage 6. Stage 6. Recovery of %BS This stage is one in which the sulfur budget of the terrestrial and aquatic systems is in a steady state with respect to the constant rates of SO4 deposition. The changes occurring during this stage are an increase in the %BS of
the terrestrial system and a recovery of BCs in surface waters because the BCs supplied by primary weathering now exceed adsorption demands
• Stage 7. Stable period of lake recovery. The systems are now in steady state with the lower levels of S04“ deposition.
Long-term depletion of calcium and other nutrients in eastern US forests
C. Anthony Federer et al., 1989 Environmental Management volume 13, pages593–601 “Both harvest removal and leaching losses can deplete nutrient capital in forests, but their combined long-term effects have not been assessed previously. We estimated changes in total soil and biomass N, Ca, K, Mg, and P over 120 years from published data for a spruce-fir site in Maine, two northern hardwood sites in New Hampshire, central hardwood sites in Connecticut and Tennessee, and a loblolly pine site in Tennessee. For N, atmospheric inputs counterbalance the outputs, and there is little long-term change on most sites. For K, Mg, and P, the total pool may decrease by 2%–10% in 120 years depending on site and harvest intensity. For Ca, net leaching loss is 4–16 kg/ha/yr in mature forests, and whole-tree harvest removes 200–1100 kg/ha. Such leaching loss and harvest removal could reduce total soil and biomass Ca by 20%–60% in only 120 years. We estimated unmeasured Ca inputs from rock breakdown, root-zone deepening, and dry deposition; these should not be expected to make up the Ca deficit. Acid precipitation may be the cause of current high leaching of Ca. Although Ca deficiency does not generally occur now in acid forest soils, it seems likely if anthropogenic leaching and intensive harvest removal continue.
Mechanisms of base-cation depletion by acid deposition in forest soils of the northeastern U.S.
Gregory B. Lawrence et al., 1999. In Sugar maple ecology and health: proceedings of an international symposium; 1998 June 2-4; Warren, PA. Gen. Tech. Rep. NE-261. Radnor, PA: U.S. Department of Agriculture, Forest Service, Northeastern Research Station: 75-87.
SOIL-CALCIUM DEPLETION LINKED TO ACID RAIN AND FOREST GROWTH IN THE EASTERN UNITED STATES
USGS 1999 A more widely readable version of Lawrence et al., 1999.
| Effects on fish (papers 1970-2000)
Carl L. Schofeld, 1974. Acid Precipitation: Effects on Fish. Ambio , 1976, Vol. 5, No. 5/6 (1976), pp. 228-230 Short Summary: Rapid extinction rates of fish populations inhabiting acidified waters have been observed over the past few decades in the Scandinavian countries and in parts of eastern North America. Extinction is often a result of chronic reproductive failure due to acidification induced effects during sensitive stages of the life-cycle [All references cited below in Schofueld 1974 are to papers published 1972-1976, except (5) which is to a 1926 paper by K Dahl, Tidskr,ftjfor Det Norske Landbruk 33, 23] EXTENT AND TRENDS OF FISH DEPLETION Declining fish populations in dilute lakes and streams exposed to acid precipitation inputs have been reported in Norway (1), Sweden (2), Canada (3), and the United States (4). In all cases, the affected waters are characterized by inherently low acid neutralizing capacity and the drainage systems are situated in areas of crystalline or metamorphic bedrock with shallow, acid soils of low buffer capacity. These conditions are pre- valent in the southern regions of Norway and Sweden, the mountainous areas of the northeastern USA, and the pre- Cambrian shield in eastern Canada. The fish fauna inhabiting these waters vary considerably in species composition; how ever, the communities are ecologically similar and are usually dominated by a salmonid-coregonid species complex in undisturbed states. Canada: The first reports in North America linking lake acidification and the extinction of fish populations to acid precipitation were derived from studies of lakes in the vicinity of Sudbury, Ontario (12-16). Populations of lake trout (Salvelinus namaycush), lake herring (Coregonus artedii), white suckers (Catostomus commersoni) and other species disappeared rapidly during the 1960s from a group of remote lakes in the LaCloche Mountain region, some 65 km distant from Sudbury (12). The rapid acidification and fish population extinction in this sensitive region was attributed to the spread of acid deposition from the metal smelters in Sudbury, which were emitting 2.4 million metric tonnes of sulfur dioxide annually and had recently increased stack height significantly. Increases in acidity of more than one hundredfold in the past decade were observed in some lakes and of 150 lakes surveyed, 33 were classified as “critically acidic” (pH less than 4.5) and 37 were described as “endangered” (pH 4.5-5.5). Subsequent consideration of the pH levels observed to affect fish repro- duction in the lakes of this region indicated that even lower acidity levels should be ascribed to these categories for themaintenance of successful reproduction of the most sensitivespecies (13). Baker, J.P., and C.L. Schofield. 1982. Aluminum toxicity to fish in acidic waters. Water Air Soil Pollut. 18: 289-309 “Aluminum toxicity varied with both pH and life history stage. At low pH levels (4.2 to 4.8), the presence of Al (up 0.2 mg l−1 for white suckers; 0.5 mg l−1 for brook trout) was beneficial to egg survival through the eyed stage. In contrast, Al concentrations of 0.1 mg l−1 (for white suckers) or 0.2 mg l−1 (for brook trout) and greater resulted in measurable reductions in survival and growth of larvae and postlarvae at all pH levels (4.2 to 5.6). Aluminum was most toxic in over-saturated solutions at pH levels 5.2 to 5.4. The simultaneous increase in Al concentration with elevated acidity must be considered to accurately assess the potential effect of acidification of surface waters on survival of fish populations.” Organic versus anthropogenic acidity in tributaries of the Kejimkujik watersheds in western Nova Scotia, A summary of the impact of acid rain on atlantic salmon (Salmo salar) in Canada Characteristics of three acidic lakes in Kejimkujik National Park, Nova Scotia, Canada Effects of low environmental pH on Atlantic salmon... Acid Toxicity Levels in Nova Scotian Rivers Have Not Declined in Synchrony with the Decline in Sulfate Levels |
SULFUR DIOXIDE EMISSIONS
In Canada and the US, those emissions increased continuously as fossil fuel consumption increased, until about 1970, then began to decline as various controls, international Agreements were put in place.
Early Chronology
From Acid Rain. Alan Nixon, Thomas Curran. 1979. Last Revision in 1998. Science and Technology Division of the Parliamentary Research Branch

Data from Smith et al., 2010, Anthropogenic sulfur dioxide emissions: 1850–2005. Atmos. Chem. Phys. Discuss., 10, 16111–16151,
1950s – The acidification of lakes was described in Canada for the first time in the Killarney area near Sudbury in Ontario.
1976 – The Canadian Network for Sampling Precipitation (CANSAP) began monitoring rainfall in 1976.
9-11 July 1979 – The Great Lakes Science Advisory Board warned that aquatic and terrestrial ecosystems in the Great Lakes Basin were being threatened by acid rain.
15 October 1979 – The Governments of Canada and the United States jointly released the first report of the United States-Canada Research Consultation Group (RCG) on the Long-Range Transport of Air Pollutants (LRTAP). The report recognized acidic precipitation as a problem of great common concern.
13 November 1979 – Canada, the United States, and the other 32 member nations of the Economic Commission for Europe signed the international “Convention on Long-Range Transboundary Air Pollution (LRTAP)“. The signatories agreed to exchange data on sulphur dioxide emissions and on long-range industrial policies likely to affect these emissions. The Convention lacks an enforcement mechanism and does not compel the signatories to effect abatement procedures.
SOME HISTORY post 2000
2001
ACID RAIN REVISITED Advances in scientific understanding since the passage of the 1970 and 1990 Clean Air Act
Driscoll, C.T et al., 2001 Hubbard Brook Research Foundation
Widely cited overview written for non-specialists. “The absence of fish and the presence of aluminum in lakes provides important information about the condition of soils within a watershed. The release of aluminum from the soil into rivers and streams usually indicates that the available calcium in the soil is low and has been depleted. Furthermore, trees growing in such soils may experience greater nutritional stress….Terrestrial recovery is even more difficult to project than aquatic recovery. Given the life span of trees and the delay in the response of soil to decreases in acid deposition, it is reasonable to suggest that decades will be required for affected trees on sensitive sites to recover once chemical conditions in the soil are restored. The time required for chemical recovery varies widely among ecosystems in the Northeast, and is primarily a function of:
– the historic rate of sulfur and nitrogen deposition;
-the rate and magnitude of decreases in acid deposition;
– the extent to which base cations such as calcium have been depleted from the soil;
-the extent to which sulfur and nitrogen have accumulated in the soil and the rate at which they are released as deposition declines;
– the weathering rate of the soil and underlying rock and the associated supply of base cations to the ecosystem; and
– the rate of atmospheric deposition of base cations.
Overall, the timing and extent of chemical and biological recovery depend
on how soon and to what extent emissions that cause acid deposition are reduced.”
2002
Health of Eastern North American Sugar Maple Forests and Factors Affecting Decline
Stephen B. Horsley et al., 2002. Northern Journal of Applied Forestry, Vol. 19, No. 1, “While sugar maple generally is healthy throughout its range, decline disease of sugar maple has occurred sporadically during the past four decades…. declines seemed to be more common on base poor soils. In a study beginning in 1985…l iming increased soil pH and exchangeable Ca and Mg in the upper horizons, while exchangeable A1 and Mn decreased. After a lag of 3-8 yr, there were significant increases in survival, crown vigor, diameter and basal area growth, and flower and seed crop production for sugar maple on limed compared with unlimed areas. None of these benefits occurred for American beech or black cherry trees at the same sites. High levels of nitrate and sulfate deposition received in northwestern Pennsylvania contribute to low Mg and Ca availability because they accelerate base cation loss ”
2003
Acidic deposition, nutrient leaching and forest growth
George H. Tomlinson in Biochemistry 65, pages51–81 (2003)
Good overview
2006
Base cation reservoirs in soil control the buffering capacity of lakes in forested catchments
Daniel Houle et al., 2006. Can. J. Fish. Aquat. Sci. 63: 471–474 Abstract: The acidification of forest soils and surface waters and their relatively poor recovery record following reduc- tions in atmospheric sulphur emissions is a major ongoing environmental problem, particularly in northeastern North America. The slow recovery of surface water is widely hypothesized to result from depletion of reservoirs of base cat- ions in soil. This is concordant with the theory that the acid-neutralizing capacity (ANC) of lakes is likely proportional to the size of the exchangeable base cation reservoirs present in surrounding watershed soils. However, data describing these linkages are still nonexistent in the literature. Here we show that lake ANC is highly predictable (r2 = 0.75) based on the size of the exchangeable Ca2+ reservoir in soil in 21 catchments representative of soil and lake conditions encountered in northeastern North America. This finding indirectly supports the hypothesis that the poor recovery of surface water from acidification is governed by the size of base cation reservoirs present in catchment soils. The size of the base cation reservoir in soil is thus a strong indicator of the acid–base status of both soils and surface waters.
A comparison of weathering rates for acid-sensitive catchments in Nova Scotia, Canada and their impact on critical load calculations
C.J.Whitfield et al., 2006.Geoderma Volume 136, Issues 3–4, 15 December 2006, Pages 899-911
“Critical loads are strongly dependent on the rate of release of base cations from the soil matrix. This study compares five commonly used methods for estimating weathering rates at five acid-sensitive catchments across Nova Scotia, Canada. Three of the methods (Zr Depletion, Clay Content, and the PROFILE model) are based on soil profiles and consider only the rooting zone, whereas the two remaining methods (the soil acidification model MAGIC and catchment Mass Balance Deficit) are catchment-based, and account for contributions from all soils within a catchment. Each weathering estimate method resulted in similar values among the five catchments, indicating similar sensitivity to acidic deposition among the study areas. Base cation weathering estimates were very low using the three soil profile-based methods, with rates varying from 3 to 13 mmolc m− 2 a− 1. In contrast, catchment-based methods predicted base cation weathering rates an order of magnitude higher (60 to 155 mmolc m− 2 a− 1), possibly due to spatial heterogeneity of the soil deposits, and contributions from deeper soil (till). Critical load (sulphur and nitrogen) estimates using the profile-based weathering rates indicate that critical loads for forest soils are currently exceeded at all catchments by 23 to 61 mmolc m− 2 a− 1.Predicted future reductions in acidic deposition should reduce the magnitude of critical load exceedance, but will not result in the catchments reaching a non-exceeded state.”
2007
-
- Freshwater acidification research in Atlantic Canada: a review of results and predictions for the future
TA Clair et al., 2007.
ABSTRACT
Atlantic Canada receives the lowest acid deposition amounts in eastern North America, but has some of the most acidic surface waters on the continent, due to the low buffering provided by regional bedrock and wetlands that produce natural organic acids. Southwestern and eastern parts of Nova Scotia combine poor buffering, high organic acidity, and higher acid deposition, to produce extremely low surface water pH and acid neutralization capacity (ANC) values. Although sulfate deposition is decreasing, concurrent reductions in dissolved base cations, as well as the acid-base characteristics of natural organic acids, are not allowing the recovery of ANC or surface water pH. Spring-time acid pulses occur in Atlantic Canada, though these have been reduced in severity with decreases in winter acid deposition, while autumnal low pH pulses caused by organic acids are a regular occurence in Nova Scotia and must be separated from mineral acidity pulses. Geochemical modeling using both critical load and dynamic approaches, nevertheless predict improvements in the water chemistry of Nova Scotia lakes within the next 20 years. However, re-establishment of pre-acidification water chemistry in most of its lakes will require greater reductions in S emissions than are currently planned in Canada and the United States.
 :
- Freshwater acidification research in Atlantic Canada: a review of results and predictions for the future
- Mapping Forest Sensitivity to Atmospheric Acid Deposition. 2007. Conference of New England Governors and Eastern Canadian Premiers Forest Mapping Group Report. 12pp.
Summary
The Forest Mapping Work Group has undertaken to map the sensitivity of the entire New England Governor and Eastern Canadian premier (NEG/ECP) jurisdictions’forests to atmospheric sulfur and nitrogen deposition loadings. Unprecedented for level of detail and size of area studied, this comprehensive project is the first scientific large-scale study of forest sensitivity to sulfur and nitrogen deposition in northeastern North America.…[from the text]The critical load map developed for the NEG/ECP region is derived on the basis of steady-state or static models. Consequently the map reflects conditions of nutrient balance rather than absolute measures of soil acidity/fertility. Nevertheless one might observe that where a negative nutrient imbalance is small, forest health problems and growth decline may not yet be evident; in those locations where the imbalance is significant, the impacts on forest health are likely to be observable today.
…The Critical Load mapping performed for the Region indicates that up to 61% of forested land within certain of the region’s jurisdictions have been characterized as ‘sensitive’, and thus may be experiencing a net nutrient deficit and increasing soil acidification. Soil mineral nutrient depletion has been linked to a wide variety of forest health problems, including reduced growth rates and increased mortality.
…Research has shown that sensitive forest areas show losses in forest health and productivity, and are more susceptible to climatic stress events, pests and diseases. For instance, preliminary results from the 30 sites of the Quebec Forest Monitoring Network (RESEF) show that forest sites in sensitive areas are growing 30% more slowly than sites located in tolerant areas.
2008
Key interactions between nutrient limitation and climatic factors in temperate forests: a synthesis of the sugar maple literature
Samuel B. St.Clair et al., 2008. Canadian Journal of Forest Research “Mineral stress (nutrient deficiency and (or) ion toxicity) is a widespread phenomenon in forests around the world. However, with the exception of N limitation, its significance is often under appreciated. On weathered, acidic soils that support many of the world’s forests, P, Ca, and Mg deficiencies and toxicities of Al and Mn are important constraints to forest productivity. Nutrient resources are a primary controller of forest function and structure and have important trophic implications, because foliar nutrient status is an important determinant of leaf palatability and consumer fitness. Nutrient acquisition and utilization in forest ecosystems is strongly influenced by environmental factors, which are changing at unprecedented rates with regional and global climate shifts. Here we examine nutrient limitations common to temperate, sugar maple (Acer saccharum Marsh.) dominated forests as a model for understanding how climatic factors influence the acquisition and utilization of nutrient resources in forest ecosystems. In general, foliar nutrient imbalances created by soil weathering and acidification impair sugar maple physiology and correlate with health decline symptoms. Extremes in light environment, temperature, precipitation, pathogen attack, and herbivory tend to induce and (or) negatively interact with nutrient imbalances in sugar maple. A conceptual model is presented that characterizes abiotic and biotic interactions influencing sugar maple health and fitness in the context of nutrient limitation.”2010
COSEWIC Assessment Summary – November 2010 Atlantic Salmon – Nova Scotia Southern Upland population. Status: Endangered. Reason for designation: Acidification of fresh water in eastern Canada is primarily a result of depositions of airborne pollutants originating in the central U.S. and Canada, though inputs are augmented by local sources as well (DFO 2000). Currently, acid impacts on Atlantic Salmon are most pronounced in the Southern Upland region of Nova Scotia (DU 14) where 22% of rivers are acidified and have lost populations and a further 31% are moderately impacted by acidification and maintain remnant populations (DFO 2000). Assuming a smolt-to-adult return rate of 5%, a value higher than is presently being observed, acidification impacts will likely result in the extirpation of 85% of the Southern Upland populations. The underlying geology of the Southern Upland is the principle reason for the vulnerability to acidification.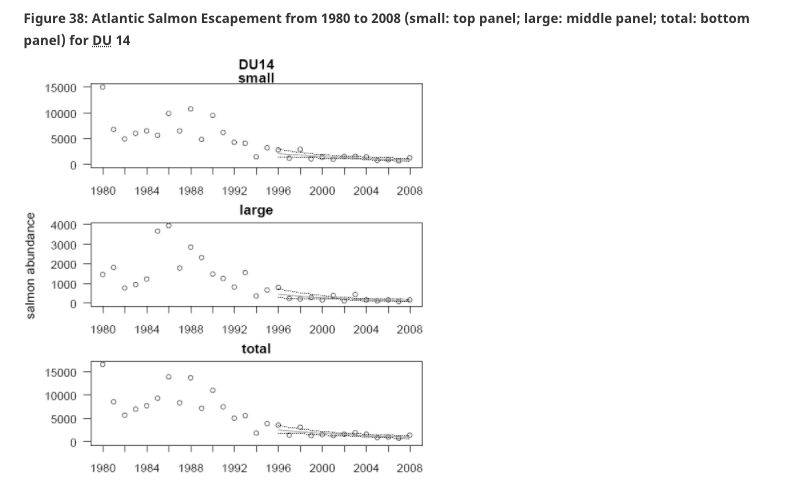 Acidification of fresh water in eastern Canada is primarily a result of depositions of airborne pollutants originating in the central U.S. and Canada, though inputs are augmented by local sources as well (DFO 2000). Currently, acid impacts on Atlantic Salmon are most pronounced in the Southern Upland region of Nova Scotia (DU 14) where 22% of rivers are acidified and have lost populations and a further 31% are moderately impacted by acidification and maintain remnant populations (DFO 2000). Assuming a smolt-to-adult return rate of 5%, a value higher than is presently being observed, acidification impacts will likely result in the extirpation of 85% of the Southern Upland populations. The underlying geology of the Southern Upland is the principle reason for the vulnerability to acidification.Other areas in Atlantic Canada that are somewhat vulnerable to the effects of acid depositions are southwestern and northeastern Newfoundland (Environment Canada 2004). Although there has been a reduction in sulphate emissions and depositions, there has not been a corresponding increase in pH or acid neutralizing capacity in these areas. Furthermore, at the projected sulphate deposition rates, the time for recovery of base cations in these catchments is 60-80 years (Clair et al. 2004). Based on the cumulative effects and extirpations, the estimated time to recovery for affected drainages, and the large area affected, acidification remains a significant threat to one DU (14, Nova Scotia Southern Upland) and is a burden if not a threat to perhaps one other (DU 4) in Newfoundland.
Acidification of fresh water in eastern Canada is primarily a result of depositions of airborne pollutants originating in the central U.S. and Canada, though inputs are augmented by local sources as well (DFO 2000). Currently, acid impacts on Atlantic Salmon are most pronounced in the Southern Upland region of Nova Scotia (DU 14) where 22% of rivers are acidified and have lost populations and a further 31% are moderately impacted by acidification and maintain remnant populations (DFO 2000). Assuming a smolt-to-adult return rate of 5%, a value higher than is presently being observed, acidification impacts will likely result in the extirpation of 85% of the Southern Upland populations. The underlying geology of the Southern Upland is the principle reason for the vulnerability to acidification.Other areas in Atlantic Canada that are somewhat vulnerable to the effects of acid depositions are southwestern and northeastern Newfoundland (Environment Canada 2004). Although there has been a reduction in sulphate emissions and depositions, there has not been a corresponding increase in pH or acid neutralizing capacity in these areas. Furthermore, at the projected sulphate deposition rates, the time for recovery of base cations in these catchments is 60-80 years (Clair et al. 2004). Based on the cumulative effects and extirpations, the estimated time to recovery for affected drainages, and the large area affected, acidification remains a significant threat to one DU (14, Nova Scotia Southern Upland) and is a burden if not a threat to perhaps one other (DU 4) in Newfoundland.2009
Present-day expansion of American beech in northeastern hardwood forests: Does soil base status matter?
Louis Duchesne and Rock Ouimet. 2009. Canadian Journal of Forest ResearchVolume 39, Number 12 “Recently, sugar maple (Acer saccharum Marsh.) decline in northeastern North America has been regarded as a major factor structuring hardwood forests by favouring American beech (Fagus grandifolia Ehrh.) in the understory of maple-dominated stands…, this study supports the hypothesis that soil base cation depletion, caused in part by atmospheric acid deposition, is among the main factors involved in the present-day expansion of American beech over a large area in Quebec.”Habitat may limit herb migration at the northern edge of the Appalachian deciduous forest
N. Hill and D. Garbury 2011 in Botany Vol 89 “Forest herbs account for greater species richness than any other plant type in deciduous forests and are the most vulnerable to anthropogenic disturbances. We examined whether the limited distribution of rare Appalachian forest herbs in Nova Scotia is related to edaphic specialization or a history of anthropogenic disturbance. Remnant populations are restricted to floodplain forest, where both habitat factors and disturbance history differ significantly from those of adjacent upland sugar maple forest. Contrasting soil and litter layers between floodplain stands and adjacent upland sites revealed the latter to be deficient in key cations (calcium, magnesium, boron); however, regression models for uplands and for floodplains showed that native herb richness was related to soil fertility in each case. Soil calcium accounted for most of the species richness variation among floodplains for native herbs and for a large seeded guild that contains most of the rare species on floodplains. Given the widespread anthropogenic decalcification of forest soils throughout eastern North America, conservation efforts must (i) increase and connect deciduous forest floodplain ecosystems and (ii) understand how to manage and create suitable cation-rich migration corridors in the forest landscape.”2014
Rates of sustainable forest harvest depend on rotation length and weathering of soil minerals
Matthew A.Vadeboncoeur et al., Forest Ecology and Management
Volume 318, 15 April 2014, Pages 194-205 “Highlights
•Organic matter and primary minerals supply nutrients over multiple rotations.
•Sustainability of long-term bioenergy harvesting is dependent on rotation length.
•Intensive short-rotation harvesting could rapidly deplete soil nutrient capital.
•Current BMPs are unlikely to severely deplete soil fertility in the 21st century.
•Over the longer term, fertilization will be necessary to balance nutrient budgets….”Harvest-induced leaching accounts for only about 20% of total rotation Ca losses under the whole-tree harvesting scenario; the bulk of nutrient capital exported each rotation is in the biomass.2015
Ecological benefits and risks arising from liming sugar maple dominated forests in northeastern North America
Jean-David Moore et al., 2015. Environmental ReviewsVolume 23, Number 1, March 2015 “Liming, the application of carbonate materials (e.g., CaCO3, CaMg(CO3)2) to soils and surface waters, has been used extensively in Europe, and to a lesser extent in Canada and the United States, to mitigate the effects of acid deposition on forest and aquatic ecosystems. This literature review… Based on current scientific literature, it is not anticipated that liming at rates of 1–3 t ha−1 would have major detrimental effects on these ecosystems. However, liming could have negative effects on northern hardwood forests with regard to earthworm invasions. The choice of liming as a mitigation tool should be made not only after weighing the potentially negative effects against the benefits of restoring sugar maple dominated stands in poorly buffered soils, but also after considering ecological components that could be lost or never recovered if an acidified forest ecosystem is not limed.Declining Acidic Deposition Begins Reversal of Forest-Soil Acidification in the Northeastern U.S. and Eastern Canada
Gregory B. Lawrence et al., 2015. Environ. Sci. Technol. 2015, 49, 13103−13111 “Decreases of exchangeable Al in the O horizon and increases in pH in the O and B horizons were seen at most sites. Among all sites, reductions in SO42− deposition were positively correlated with ratios (final sampling/initial sampling) of base saturation (P < 0.01) and negatively correlated with exchangeable Al ratios (P < 0.05) in the O horizon. However, base saturation in the B horizon decreased at one-third of the sites, with no increases. These results are unique in showing that the effects of acidic deposition on North American soils have begun to reverse.Wood Decay Fungi Restore Essential Calcium to Acidic Soils in Northern New England
by Walter C. Shortle * andKevin T. Smith Forests 2015, 6(8), 2571-2587A PERSPECTIVE IN 2016 A critical issue: nutrient depletion in soils of Nova Scotia’s forests
Overview in April, 2016. Comments and diagram were prepared for meetings with Premier and MLAs, re: clearcutting for biomass electricity. View 2016 Document
Map shows Critical land Exceedances (in mole-equivalents) for the Maritime provinces and a portion of Maine. Map Source: Miller, E. et al. 2007. Mapping Forest Sensitivity to Atmospheric Acid Deposition. Conference of New England Governors and Eastern Canadian Premiers Forest Mapping Group Report. 12pp. Original Map shows more of NE USA, Quebec and Newfoundland. This graphic was prepared April, 2016. Insets are described in text. The “Critical Load” represents the maximum loading of acidifying sulphur and nitrogen compounds in precipitation that can be tolerated without (known) harmful effects. Positive numbers (yellow to red) indicate those levels are being exceeded. Green numbers are areas where it is not being exceeded, the darker green the more acid precipitation could be tolerated without exceedance.
Insets
A. The broad outlines of this issue have been known since the 1980s when declines in salmon on the Atlantic coast of Nova Scotia were related to acidification of surface waters, that in turn attributed to acid rain and the poor buffering capacity of the forest ecosystems, especially those developed on slates, granites and felsic bedrock.[1]
B. By the mid-2000’s, it was clear that forest soils over more than 50% of Nova Scotia’s land mass have unusually low buffering capacity in comparison to most other jurisdictions in eastern North America and are critically low in nutrients (notably calcium) essential for the health of both the forests and aquatic systems in many of our forested watersheds.[2] [Re: Map above, “the first scientific large-scale study of forest sensitivity to sulfur and nitrogen deposition in northeastern North America.”
C. 2009-2011 Nutrient modeling commissioned by the Nova Scotia Dept of Natural Resources illustrated that clearcutting increases nutrient losses substantially, thereby further threatening aquatic systems and future productivity of forests. DNR hads not released the report received in 2011, citing confidentiality concerns.[3]
D. 2011 Elsewhere in eastern North America, reductions in surface water acidity and levels of toxic aluminum have been observed following reductions in sulfur emissions.However, in SW Nova Scotia in particular, waters continue to acidify and levels of toxic aluminum to increase, which is attributed to extremely low levels of calcium.[4]
E. 2016 Clearcuts continue in the most susceptible landscapes, e.g. in the St. Margaret’s Bay ecodistrict, threatening both the future productivity and biodiversity of forests and the health of aquatic ecosystems.
Notes and References
1. E.g., Watt, W.D. 1987. A summary of the impact of acid rain on Atlantic salmon (Salmo salar) in Canada. Water Air and Soil Pollution 35:27–35.2. Miller, E. et al. 2007. Mapping Forest Sensitivity to Atmospheric Acid Deposition. Conference of New England Governors and Eastern Canadian Premiers Forest Mapping Group Report. 12pp.
3. A DNR Slide Presentation in 2009 titled Nova Scotia Forest Biomass Harvest and Retention Guidelines highlighted a “soil nutrient budget computer model–a decision support model to assess site suitability for biomass harvest in NS.DNR contracting with UNB”, which would be released mid-2010. The only publicly available document available to date from that study appears to be a thesis released later (& independently of NSDNR) by UNB: Noseworthy, J. 2011. Mass balance, biogeochemical framework for assessing forest biomass harvest sustainability. MSc thesis, University of New Brunswick. Data are presented only for Kejimkujik National Park “due to confidentiality concerns with Nova Scotia forest inventory data”. A 2014 slide presentation by a DNR soil scientist (Kevin Keys: Forest Nutrition Management in Nova Scotia Overview of some NSDNR research initiatives) describes the current state of the NBM-NS (Nutrient Budget Model for Nova Scotia) which was received in 2011. The delay is said to be due to a need to “clarify, check, and update (as needed) model components.” I was told by another DNR official in the fall of 2015 that it could be another 5 years before the model is ready for use.
4. Clair, T.A. et al. 2011. Water chemistry and dissolved organic carbon trends in lakes from Canada’s Atlantic Provinces: no recovery from acidification measured after 25 years of lake monitoring Canadian Journal of Fisheries and Aquatic Sciences . 68: 663–674.
Effects of acidification on aquatic biota in Atlantic Canada
P Lacoul et al., 2011 Environmental ReviewsDennis, I.F. ,& T.A. Clair. 2012. The distribution of dissolved aluminum in Atlantic salmon (Salmo salar) rivers of Atlantic Canada and its potential effect on aquatic populations. Canadian Journal of Fisheries and Aquatic Sciences69:1174-1183.
5. [COMMENT] Under DNR’s developing NBM-NS (cited in note 3 above), sustainability would be assessed at the stand level with “sustainability assessment… based on whether estimated primary nutrient inputs are ≥ primary nutrient outputs under each harvest scenario over the long-term, and on whether current base saturation levels have been maintained.” Such a scheme would not allow harvesting of the most nutrient stressed stands which reduces risks to their future productivity. However it would, for example, allow harvesting on a nutrient rich drumlin within an otherwise highly acidic watershed which could threaten localized populations of brook trout or salmon as well as accentuate acidification of all downstream waters. Banning all clearcuts within a highly acidic watershed would be necessary to protect both future forest productivity and aquatic ecosystem health. It would also simplify the decision-making process and could be implemented immediately as the necessary information is already available for most watersheds. Such an approach would benefit from, if not require, more integration of activities related to soil and water acidification within government departments (Environment at both federal and provincial levels, NS DNR, NS Fisheries and Aquaculture) as well as with researchers at universities.
6. “Water chemistry conditions suitable to allow the survival and thriving of Atlantic salmon, the most visible symbol of the acidification problem in much of Nova Scotia, have not improved in the past 30 years. Geochemical modeling and theory suggest that they can only recover under lower acid deposition levels than are currently being endured and after seveal decades of natural weathering to allow base cation replenishment of soils from resistant bedrock.” – Clair et. Al, 2011 (cited in note 4 above).
2016
Natural and anthropogenic drivers of calcium depletion in a northern forest during the last millennium
Bérangère A. Leys et al., 2016. PNAS vol. 113 | no. 25. “Significance: This research breaks new ground by showing that, contrary to generally accepted theories of ecosystem development, calcium depletion has been occurring for millennia as a natural consequence of long-term ecosystem development. This natural process predisposed forest ecosystems in the region to detrimental responses to acid rain in the 20th century. We also
show that nitrogen availability was increasing concurrently with the depletion of calcium. This is the first study, to our knowledge, to reconstruct continuous changes in nutrient availability for a northern forest ecosystem on the millennial time scale. The results alter our assessments of the speed and trajectory of nutrient limitation in forests and suggest that reformulation of global models of forest productivity may
be necessary2018-2021
Long-term decline of sugar maple following forest harvest, Hubbard Brook Experimental Forest, New Hampshire
Natalie L. Cleavit et al., 2018. Canadian Journal of Forest Research “Forest harvesting can impact site quality by removing essential nutrients, exacerbating the effects of historic base cation losses associated with acid deposition…The results support previous studies indicating that regeneration by sugar maple is severely compromised on base cation depleted soils. Lower survival of seedlings for sugar maple emphasized the importance of maintaining advance regeneration to favor desired species such as sugar maple. Foresters should consider that sites with low base saturation and exchangeable Ca are likely to exhibit regeneration failure for sugar maple in the long term, even those with initial dominance by this species.”Increasing biomass demand enlarges negative forest nutrient budget areas in wood export regions
Wagner de Oliveira Garcia et al., 2018. Nature Scientific Reports volume 8, Article number: 5280
“Energy production from biomass is one of the adopted strategies in different European countries to limit global warming to within the 1.5–2° targets after the 2015 UN climate agreement. This will motivate enhanced forest harvest rates and whole tree harvest to supply the increasing biomass demand. Negative nutrient budgets for certain timberland areas where geogenic nutrient supply cannot cope with harvesting rates will be one consequence. A spatially explicit analysis for a U.S. timberland area of 33,570 km2 reveals that for a minimum nutrient loss and supply scenario, negative nutrient budgets occur in 17, 20, 16, and almost 94% of the studied areas for Ca, K, Mg, and P, respectively. For a maximum nutrient loss (considering intensive harvesting) and supply assumptions, the affected areas increase to 50, 57, 45 and 96% for Ca, K, Mg, and P, respectively. In general, atmospheric nutrient deposition is of minor importance for the high weathering supply cases. Increasing global woody biomass demand may cause additional pressure on forested ecosystems, enlarging negative nutrient budget areas. If woody biomass demand rises, strategies to counterbalance nutrient gaps might be needed, for example, by preparing harvested areas with rock products, designed to replenish growth limiting nutrients, and/or implementing forest management strategies to minimize nutrient export.”The response of soil solution chemistry in European forests to decreasing acid deposition
Johnson et al., 2018 in Global Change Biology “Abstract Acid deposition arising from sulphur (S) and nitrogen (N) emissions from fossil fuel combustion and agriculture has contributed to the acidification of terrestrial ecosystems in many regions globally. However, in Europe and North America, S deposition has greatly decreased in recent decades due to emissions controls. In this study, we assessed the response of soil solution chemistry in mineral horizons of European forests to these changes. Trends in pH, acid neutralizing capacity (ANC), major ions, total aluminium (Altot) and dissolved organic carbon were determined for the period 19952012. Plots with at least 10 years of observations from the ICP Forests monitoring network were used. Trends were assessed for the upper mineral soil (1020 cm, 104 plots) and subsoil (4080 cm, 162 plots). There was a large decrease in the concentration of sulphate (urn:x-wiley:13541013:media:gcb14156:gcb14156-math-0001) in soil solution; over a 10-year period (20002010), urn:x-wiley:13541013:media:gcb14156:gcb14156-math-0002 decreased by 52% at 1020 cm and 40% at 4080 cm. Nitrate was unchanged at 1020 cm but decreased at 4080 cm. The decrease in acid anions was accompanied by a large and significant decrease in the concentration of the nutrient base cations: calcium, magnesium and potassium (Bc = Ca2+ + Mg2+ + K+) and Altot over the entire dataset. The response of soil solution acidity was nonuniform. At 1020 cm, ANC increased in acid-sensitive soils (base saturation ¾10%) indicating a recovery, but ANC decreased in soils with base saturation >10%. At 4080 cm, ANC remained unchanged in acid-sensitive soils (base saturation ¾20%, urn:x-wiley:13541013:media:gcb14156:gcb14156-math-0003 ¾ 4.5) and decreased in better-buffered soils (base saturation >20%, urn:x-wiley:13541013:media:gcb14156:gcb14156-math-0004 > 4.5). In addition, the molar ratio of Bc to Altot either did not change or decreased. The results suggest a long-time lag between emission abatement and changes in soil solution acidity and underline the importance of long-term monitoring in evaluating ecosystem response to decreases in deposition.”Widespread diminishing anthropogenic effects on calcium in freshwaters
Gesa A. Weyhenmeyer et al., 2019 Nature Scientific Reports 9, Article number: 10450
Calcium (Ca) is an essential element for almost all living organisms. Here, we examined global variation and controls of freshwater Ca concentrations, using 440 599 water samples from 43 184 inland water sites in 57 countries. We found that the global median Ca concentration was 4.0 mg L−1 with 20.7% of the water samples showing Ca concentrations ≤ 1.5 mg L−1, a threshold considered critical for the survival of many Ca-demanding organisms. Spatially, freshwater Ca concentrations were strongly and proportionally linked to carbonate alkalinity, with the highest Ca and carbonate alkalinity in waters with a pH around 8.0 and decreasing in concentrations towards lower pH. However, on a temporal scale, by analyzing decadal trends in >200 water bodies since the 1980s, we observed a frequent decoupling between carbonate alkalinity and Ca concentrations, which we attributed mainly to the influence of anthropogenic acid deposition. As acid deposition has been ameliorated, in many freshwaters carbonate alkalinity concentrations have increased or remained constant, while Ca concentrations have rapidly declined towards or even below pre-industrial conditions as a consequence of recovery from anthropogenic acidification. Thus, a paradoxical outcome of the successful remediation of acid deposition is a globally widespread freshwater Ca concentration decline towards critically low levels for many aquatic organisms.Assessing and Monitoring Soil Productivity, Carbon Storage, and Conservation on the Maine Adaptive Silviculture Network
Joshua Puhlick et al., 2020 in Cooperative Forestry Research Unit Annual Report, p51-57 ‘…Sauls Brook had lower Ca and Mg stocks compared with Seven Islands, which likely contributed to the higher percentage of American beech and lower percentage of sugar maple at Sauls Brook. There were also significant differences in the effective base saturation in the upper B horizon between installations, with the Sauls Brook installation having values shown to adversely affect sugar maple. Hence, soil properties will be drivers of future species composition and carbon trajectories, and these trajectories will likely vary by specific harvest areas across the landscape. Non-native earthworms To our surprise, we discovered non-native earthworms at the Nashville Plantation installation.Non-native earthworms invade forest soils in Northern Maine, USA.
Puhlick, J. J., I. J. Fernandez, and J. W. Wason. 2021. Forests. 12, 80. doi: 10.3390/f12010080
Tamm Review: Nutrient cycling in forests: A historical look and newer developments
Dale W.Johnson & JohnTurner Dale W.JohnsonaJohnTurner Forest Ecology and Management Volume 444, 15 July 2019, Pages 344-373 “Abstract In this review, we consider a traditional conceptual model of nutrient cycling in forests and evaluate (1) assumptions and issues with existing methods for measuring and calculating nutrient pools and fluxes, including the estimation of errors; (2) how various elements of the conceptual model vary with geographic and climatic region, and gaps in knowledge about certain regions; (3) predictions from nutrient cycling data for the effects of harvesting, burning, fertilization, and elevated CO2, including the effects of nutrient cycling on productivity and the effects of productivity on nutrient cycling. As is true of all models, traditional models of forest nutrient cycling are all incorrect in the sense that they are approximations and do not capture all features the real world. For example, none of these traditional models include the important effects of catastrophic events such as wildfire, insect attack, hurricanes, etc. Nonetheless, traditional nutrient cycling models have allowed us to explore the collective implications of our current understanding of nutrient cycling processes. While the methods apply to plantations the focus of this review has been on natural forest studies. Despite much effort, reliable estimates of some transfers such as soil weathering and nitrogen fixation remain elusive. Soluble exports on a watershed level are not reliable representatives of exports from terrestrial nutrient cycles because correct conditions for such measurements are relatively rare and also because such estimates are subject to deep soil weathering and stream spiraling beyond the rooting zone. Soluble exports by lysimetery are subject to errors in the estimation of water flux and the delineation of the depth of rooting. The current versions of these traditional models will no doubt require modifications in the future to account for new information becomes available, for example, the delays between root uptake and the appearance of nutrients in aboveground biomass, the importance of soil nutrient hotpots for uptake, and the unforeseen ability of nitrogen-limited trees to extract additional nitrogen from soils when root growth in stimulated by elevated CO2.”Conventional analysis methods underestimate the plant-available pools of calcium, magnesium and potassium in forest soils
Jérémie Bel et al., 2020 NatureAtmospheric deposition and exceedances of critical loads from 1800 2025 for the conterminous United States
CHRISTOPHER M. CLARK et al., 2018. Ecological Applications, 28(4), 2018, pp. 978–1002 “Atmospheric deposition of nitrogen (N) and sulfur (S) has increased dramatically over pre-industrial levels, with many potential impacts on terrestrial and aquatic ecosystems. Quantitative thresholds, termed “critical loads” (CLs), have been developed to estimate the deposition rate above which damage is thought to occur. However, there remains no comprehensive comparison of when, where, and over what time periods individual CLs have been exceeded…In total, it is clear that many CLs have been exceeded for decades, and are likely to remain so in the short term under current policies. Additionally, we suggest many areas for improvement to enhance our under- standing of deposition and its effects to support informed decision making.”A century of change: Reconstructing the biogeochemical history of Hubbard Brook
Gene E. Likens et al., 2021 in Hydrological Processes. “Ecosystems constantly adjust to altered biogeochemical inputs, changes in vegetation and climate, and previous physical disturbances. Such disturbances create overlapping ‘biogeochemical legacies’ affecting modern nutrient mass balances. To understand how ‘legacies’ affected watershed-ecosystem (WEC) biogeochemistry during five decades of studies within the Hubbard Brook Experimental Forest (HBEF), we extended biogeo- chemical trends and hydrologic fluxes back to 1900 to provide an historical framework for our long-term studies. This reconstruction showed acid rain peaking at HBEF in the late 1960s-early 1970s near the beginning of the Hubbard Brook Ecosystem Study (HBES). The long-term, parabolic arc in acid inputs to HBEF generated a corresponding arc in the ionic strength of stream water, with acid inputs generating increased losses of H+ and soil base cations between 1963 and 1969 and then decreased losses after 1970. Nitrate release after disturbance is coupled with previous N-deposition and stor- age, biological uptake, and hydrology. Sulfur was stored in soils from decades of acid deposition but is now nearly depleted. Total exports of base cations from the soil exchange pool represent one of the largest disturbances to forest and associated aquatic ecosystems at the HBEF since the Pleistocene glaciation. Because precipitation inputs of base cations currently are extremely small, such losses can only be replaced through the slow process of mineral weathering. Thus, the chemistry of stream water is extremely dilute and likely to become even more dilute than pre-Industrial Revolution estimates. The importance of calculating chemical fluxes is clearly demonstrated in reconstruction of acid rain impacts during the pre-measurement period. The aggregate impact of acid rain on WEC exports is far larger than historical forest harvest effects, and even larger than the most severe deforestation experiment (Watershed 2) at HBEF. A century of acid rain had a calcium stripping impact equivalent to two W2 experiments involving complete deforestation and herbicide applications.”
”RELATED PUBLICATIONS IN SCIENTIFIC JOURNALS 2016-2022 BY K. KEYS, SOIL SCIENTIST WITH NRR (NSDNR,L&F) - A Simple Geospatial Nutrient Budget Model for Assessing Forest Harvest Sustainability across Nova Scotia, Canada
K. Keys et al., 2016. Open Journal of Forestry 2016, 6, 420-444
This is the revised Noseworthy/Arp model of 2011 (see item 3 above). There was no accompanying announcement from DNR which had once promised that a nutrient budget model for use in Crown land harvest decisions would be “ready mid-2010” and it took a CBC Report on Nov 8, 2016 to announce that a scientific paper on the model had been published in Sept 2016. Nutrient budgeting was incorporated into the Nova Scotia Silvicultural Guide for the Ecological Matrix released July 2021, but that has yet to be applied in the field. It appears that there is no provision to allow the nutrient status to improve on soils which are severely calcium depleted because of acid rain combined with the inherently poor soils that dominate on much of our landscape. View SGEMnote - Impacts of surface applied alkaline-treated biosolids on spruce plantation soils and vegetation in Nova Scotia, Canada
Kevin Stewart Keys. PhD thesis, Dalhousie University 2018. It includes a lot of background material in more detail than in the related publications. - Forest floor chemistry and mineral soil ion exposure after surface application of alkaline-treated biosolids under two white spruce (Picea glauca) plantations in Nova Scotia, Canada
K Keys et a., 2018. Forest Ecology and Management Volume 417, 15 May 2018, Pages 208-221 Two field trials were established to evaluate the use of alkaline-treated biosolids (ATB) to offset current or predicted Ca deficits in Nova Scotia forest soils under juvenile white spruce (Picea glauca) plantations…Results suggest that ATB could be a good source of Ca in Ca-limited sites, but nutrient imbalances may be a problem on sites where K and Mg depletion has also occurred or where N is also limiting. - Ion availabilities in two forest soils amended with alkaline-treated biosolids, agricultural lime, and wood fly ash over a 10-week period
K.Keys et al., 2020. Canadian Journal of Forest Research. “Two forest soil B-horizons were amended with alkaline-treated biosolids (ATB), powdered agricultural lime, and wood fly ash under controlled conditions to compare initial ion availabilities over a 10-week period. …This suggests that ATB amendments could be an alternative means of quickly adding available Ca2+ to Ca-depleted forest soils as long as potential impacts on other nutrient base cations are considered.” - Predictive Models and Maps for the Bioeconomy
Slide Presentation by Kevin Keys June 1, 2016 to the Atlantic Biorefinery Conference
SOME RELATED RECENT PUBLICATIONS BY S. STERLING & COLLEAGUES, DALHOUSIE UNIVERSITY View this site for an overview: Sterling Hydrology and Climate Change Research Group
“Our mission is to quantify and address threats to water availability and freshwater ecosystems”Increasing aluminium concentrations in Southwest Nova Scotia Canada rivers from 1980 to present
Jeff Minichiello, Shannon Sterling, Sarah Ambrose, Tom Clair 2014. EGU General Assembly Conference Abstracts
Elevated aluminum levels in rivers is known to be toxic for aquatic species, in particular Salmo salar; however it was only recently aluminium has been identified as a potential threat to Salmo salar populations in South Western Nova Scotia, Canada (SWNS) (Dennis and Clair 2012). Previously, it was thought SWNS rivers contained enough DOC to render the aluminium in rivers inactive. A key remaining question is whether aluminium levels are declining following atmospheric pollution reductions. Here we make a first assessment of long term (1980-2011) aluminium concentration trends in three watersheds located in SWNS, as measured by weekly grab samples. Our results show that total aluminium levels have significantly increased from 1980-2011 in all three sites. Estimates of ionic aluminium levels indicate that the ionic aluminum concentration frequently exceeds the threshold for the level of aquatic health determined by the European Inland Fisheries Advisory Commission (Howells et al. 1990). Data also indicates that calcium levels have yet to recover even with declining concentrations of riverine sulfate. This new knowledge that aluminium is at toxic levels and is worsening will have implications for policy on acidification mitigation in SWNS; this is an urgent issue as the local salmon population numbers currently are declining to near extirpation levels.Widespread Increases in Iron Concentration in European and North American Freshwaters
C. Björnerås et al., 2017l In Global Biogeocheical Cycles “Recent reports of increasing iron (Fe) concentrations in freshwaters are of concern, given the fundamental role of Fe in biogeochemical processes. Still, little is known about the frequency and geographical distribution of Fe trends or about the underlying drivers. We analyzed temporal trends of Fe concentrations across 340 water bodies distributed over 10 countries in northern Europe and North America in order to gain a clearer understanding of where, to what extent, and why Fe concentrations are on the rise…”Five Aluminum Seasonality Regimes Identified in Chronically Acidified Rivers of Nova Scotia
Lobke Rotteveel and Shannon M. Sterling 2019 Environ. Sci. Technol. 2020, 54, 2, 807–817
Despite reductions in acidic deposition, high freshwater Al concentrations continue to threaten acidified ecosystems across the northern hemisphere. Seasonally elevated Al concentrations may pose a particular threat to freshwater organisms. Despite this threat, there is a lack of understanding about the timing and drivers of seasonal Al fluctuations. Here, we address this knowledge gap by identifying seasonal patterns of Al and their drivers in 16 rivers across Nova Scotia, Canada. We identify five distinct Al regimes with different timing of seasonally elevated Al concentrations. Regimes are distinguished by correlation strength and direction between Al and base cations, total organic carbon, turbidity, and discharge. Most notably, regimes are distinguished by a gradient of Al–base cation decoupling as Ca and Mg concentration approaches 1.4 mg L–1 and 0.6 mg L–1, respectively. Seasonally elevated Al concentrations exceeded the 0.1–0.2 mg L–1 World Health Organization drinking water guidelines in all regimes, and inorganic monomeric Al is projected to exceed the 15 μg L–1 threshold for aquatic health in most rivers. This research highlights the complexity of seasonal Al dynamics and the importance of understanding seasonal variation of Al to quantify the impact of Al on human health, water treatment, and aquatic organisms.Identifying global trends and drivers of freshwater aluminium concentrations using GloFAD (Global Freshwater Acidification Database)
Rotteveel, Lobke ; Sterling, Shannon 2020 22nd EGU General Assembly, held online 4-8 May, 2020, id.10854
Aluminum is toxic to most aquatic and terrestrial organisms. Increased Al concentrations in soils and freshwaters are a direct result of human activity, via increases in acid deposition. Elevated Al concentrations pose a wide variety of threats to ecosystems and society, from causing human neurotoxicity, reducing carbon sequestration in forests, threatening biodiversity, and increasing the cost of water treatment. Freshwater aluminium concentrations increased across Europe and North America between the 1960s and 1990s, predominantly due to ecosystem acidification. Following acidic deposition reduction legislation enacted in the 1990s, the problems of acidification and increased freshwater aluminium concentrations were considered solved. However, recently and unexpectedly, Sterling et al. identified aluminum concentrations to be increasing across North America and Scandinavia. Sterling et al. proposed a conceptual model suggesting these widespread increases in freshwater aluminium concentrations resulted from a hysteresis of base cation and dissolved organic carbon (DOC) response to decreasing acidic deposition, where base cation increase is slow compared to that of DOC, resulting in elevated freshwater aluminium concentrations. This process can be exacerbated by further increases in DOC due to increasing global surface temperatures…The widespread decreasing base cation trends and strong correlation between decreasing base cation and increasing aluminium trends indicates that increasing aluminium concentrations may become more widespread, posing a threat to aquatic and terrestrial organisms, potentially including humans, reducing carbon sequestration in forests, threatening biodiversity, and increasing water treatment costs.Ionic aluminium concentrations exceed thresholds for aquatic health in Nova Scotian rivers, even during conditions of high dissolved organic carbon and low flow
SM Sterling et al., 2020 Hydrology and Earth System Sciences 24 (10), 4763-4775 “Acid deposition released large amounts of aluminium into streams and lakes during the last century in northern Europe and eastern North America. Elevated aluminium concentrations caused major environmental concern due to aluminium’s toxicity to terrestrial and aquatic organisms and led to the extirpation of wild Atlantic salmon populations. Air pollution reduction legislation that began in the 1990s in North America and Europe successfully reduced acid deposition, and the aluminium problem was widely considered solved. However, accumulating evidence indicates that freshwater systems still show delays in recovery from acidification, with poorly understood implications for aluminium concentrations. Here, we investigate spatial and temporal patterns of labile cationic forms of aluminium (Ali) from 2015 to 2018 in 10 catchments in Nova Scotia, Canada; this region was one of the hardest hit by acid deposition, although it was not considered to have an aluminium problem due to its high dissolved organic carbon (DOC) concentrations that were expected to reduce Ali concentrations. Surprisingly, our results show the widespread and frequent occurrences of Ali concentrations that exceed toxic thresholds in all sampled rivers despite high DOC concentrations. Generalized linear mixed model results reveal that DOC, instead of being inversely related to Ali, is the strongest predictor (positive) of Ali concentrations, suggesting that the recruitment properties of DOC in soils outweigh its protective properties in streams. Lastly, we find that, contrary to the common conceptualization that high Ali levels are associated with storm flow, high Ali concentrations are found during base flow. Our results demonstrate that elevated Ali concentrations in Nova Scotia continue to pose a threat to aquatic organisms, such as the biologically, economically, and culturally significant Atlantic salmon (Salmo salar).”Distribution, Drivers, and Threats of Aluminum in Groundwater in Nova Scotia, Canada
KA Hart et al., 2021 Full paper in PDF “Increased rates of acid deposition derived from the burning of fossil fuels over the last century have resulted in the acidification and increase in aluminum (Al) levels in freshwaters and soils in sensitive areas. While the acidification of surface waters such as lakes and rivers has been extensively studied, the acidification status and resulting Al concentrations in groundwater are poorly understood. Here we aim to describe the distribution of Al in groundwater across the province of Nova Scotia, Canada. …. We found groundwater Al concentrations to be highest in areas underlain by plutonic and metamorphic bedrock types as well as surficial aquifers, with pH and organic carbon concentrations having the strongest correlation with groundwater Al concentrations…High concentrations of Al in groundwater may also be exported to surface waters such as rivers and lakes, where they can be harmful to aquatic populations such as Atlantic salmon (Salmo salar). We recommend that private well owners test their water supplies for Al, and that further studies on Al export from groundwater to surface water be carried out in the most high-risk areas coincident with important Atlantic salmon river watersheds.Helicopter Liming to Help Restore Acidified Forest Soil Productivity
C McCavour, S Sterling, K Keys, E Halfyard 2021 EGU General Assembly Conference Abstracts, EGU21-13660
Decades of acid deposition across northeastern North America has caused excess leaching of soil base cations (Ca2+, Mg2+, K+) and increases in bioavailable aluminum (Al3+) that, in combination, have resulted in widespread decreases in potential forest productivity. Despite major reductions in SO2 and NOx emissions since the 1990s, forest soils across the region have shown few signs of recovery from acid deposition impacts and it could take decades or centuries for natural recovery to occur. As a result, affected forests are stressed, less productive, and more prone to climate change-induced damage. Helicopter liming of upland forests may be an effective way to jump-start the soil recovery process…. These early chemical results are promising and further support the use of helicopter liming as an effective tool to combat lingering effects from acid deposition in acidified forests.Kejimkujik Calibrated Catchments: a benchmark dataset for long‐term impacts of terrestrial and freshwater acidification
Shannon Sterling,Thomas A. Clair,Lobke Rotteveel,Nelson O’Driscoll,Daniel Houle,Edmund Halfyard,Kevin Keys Hydrological Processes 12 January 2022. “Delays in forest recovery from terrestrial acidification combined with climate change are leading Acadian Forest ecosystems into new territory. The Kejimkujik Calibrated Catchments (KCC) Study Program was established in and adjacent to Kejimkujik National Park and Historic Site (KNP) in Southwest Nova Scotia (SWNS), Canada, in the late 1970s to study the impacts of acid precipitation on pristine and vulnerable ecosystems….”DIVERSE EFFECTS OF LOSS OF CALCIUM
Cited in Letter, 2014:In addition to the well known effects of calcium loss and aquatic acidification on salmonids, many studies are emerging showing that declines in calcium under forests are having diverse adverse effects either through calcium deficiency directly or indirectly through reduced pH, aluminum mobilization and enhanced mercury toxicity e.g., on cold tolerance of red spruce [11], sugar maple decline [10], forest salamanders and snails[12], loon reproduction[13], zooplankton[14], forest herbs[15], invertebrates and song birds[16]
10. Whole tree harvests at http://novascotia.ca/natr/strategy/forests/whole-tree-discussion.asp
11. M. E. Fenn et al. 2006. Status of soil acidification in North America. Journal Of Forest Science, 52 (Special Issue): 3–13.
12. C.M. Beir et al. 2012. Changes in faunal and vegetation communities along a soil calcium gradient in northern hardwood forests. Canadian Journal of Forestry Research 42: 1141–1152.
13. Bird Studies Canada. 2013. The Canadian Lakes Loon Survey
14. A. Jeziorski, et al. 2008. The widespread threat of calcium decline in fresh waters. Science 322, 1374
15. N. M. Hill & D.J. Garbary 2011 Habitat may limit herb migration at the northern edge of the Appalachian deciduous forest Botany 89: 635-645.
16. S.E. Pabian & M.C. Brittingham. 2012. Soil calcium and forest birds: indirect links between nutrient availability and community composition. Ecosystems 15: 748–760.
More… (partial list; excluding salmon)
Trout
Population density of Brown trout (Salmo trutta) in extremely dilute water qualities in mountain lakes in southwestern Norway.
Enge, E.; Kroglund, F. 2011 Water, Air, Soil Pollut.219, 489−499. We have examined populations of brown trout in low conductivity mountain lakes (5.0-13.7 mu S/cm and 0.14-0.41 mg/l Ca) in southwestern Norway during the period 2000-2010. Inlets to the lakes were occasionally even more dilute (2007; conductivity = 2.9-4.8 mu S/cm and Ca = 0.06-0.17 mg/l). The combination of pH and conductivity was the best predictor to fish status (CPUE), indicating that availability of essential ions was the primary restricting factor to fish populations in these extremely diluted water qualities. We suggest that conductivity < 5 mu S/cm is detrimental to early life stages of brown trout, and subsequently that there are lakes in these mountains having too low conductivity to support self-reproducing trout populations. Limited significance of alkalinity, Ca, Al, and color suggests that effects of ion deficit apparently overruled the effects of other parameters.Effects of Water Acidity, Calcium, and Aluminum on Whole Body Ions of Brook Trout (Salvelinus fontinalis) Continuously Exposed from Fertilization to Swim-Up: A Study by Instrumental Neutron Activation Analysis
Chris Wood et al. 2011 Canadian Journal of Fisheries and Aquatic Sciences 47(8):1593-1603 “.Water Ca, rather than pH or Al, was the most important factor affecting whole body electrolyte levels in fry exposed from fertilization to swim-up…At lower pH, this threshold was shifted to between 2 and 8 mg/L, indicating that Ca levels sufficient to support healthy development at circumneutral pH may prove inadequate under acidified conditions.” The whole text is available, reviews some older lit. “According to Schindler (1988), massive fish mortality andthe decline of salmonid fisheries are proof of the ecologicaldamage caused by acid precipitation. In Québec, atmo-spheric deposition of sulphur and nitrogen oxides is assocted with the disappearance of numerous fish populations,including several brook trout (Salvelinus fontinalis) populations…A number of authors have examined the complex interac-tions between pH, Al, and Ca2+ that must be consideredwhen attempting to determine the effects of acidification on fish (e.g., Mount et al. 1988a; Ingersoll et al. 1990a; Woodet al. 1990). Their findings reveal, in the case of brook trout,that pH < 5.2 concurrent with concentrations of filtratedAl > 200 mg·L–1 and Ca2+ < 2 mg·L–1 induces significantphysiological stress on the species’ ionoregulation and respi-ration capacity. These conditions can lead to recruitmentfailure (Haines 1981; Beggs and Gunn 1986).”Aquatic Life (General)
Effects of acidification on aquatic biota in Atlantic Canada
P. Lacoul et al., 2011 Environmental Reviews • 2 November 2011 “Acidification of surface waters is a high-profile environmental issue in Atlantic Canada. Despite a reduction of emissions of acid-precursors (particularly SO2) by more than 50% in major regions in North America, there has not yet been a significant recovery of surface waters in the region, likely because of the impoverished acid-neutralizing capacity (ANC) of watersheds. Nevertheless, any detection of a biological recovery in the region requires knowledge of acidification threshold values for indicator species, so that they can be used in an appropriate bio-monitoring program. Our review of information on the effects of acidification on aquatic organisms in Atlantic Canada suggests that the greatest changes in phytoplankton occur over a pH range of 4.7 to 5.6, just beyond the interval (pH 5.5 to 6.5) where bicarbonate (), a key source of both ANC and inorganic carbon for photosynthesis, becomes rapidly depleted and then lost. Similarly, the pH threshold of 5.5 appears to be critical to sensitive macrophytes. The pH tolerance is highly variable among invertebrate taxa, but the median tolerable pH for most sensitive species is between 5.2 and 6.1. Sensitive fish species are affected at pH levels as high as 6.0–6.5, but tolerant ones may do well even at pH <5.0. Amphibian species are relatively tolerant, surviving even to pH 3.5 to 4.0. Aquatic birds breed in the region at pH values greater than 5.5.”Widespread diminishing anthropogenic effects on calcium in freshwaters
Gesa A. Weyhenmeyer et al., 2019. Scientific Reports volume 9.” Our global analysis of Ca and carbonate alkalinity concentrations in lakes and running waters shows that Ca and carbonate alkalinity generally strongly co-vary with highest concentrations in waters with a pH between 8.0 and 9.0, and lowest concentrations in acidic water bodies. Under the influence of anthropogenic acid deposition, however, Ca concentrations became disproportionate and unnaturally high relative to alkalinity, often accompanied by higher sulfate concentrations. As acid deposition has been increasingly mitigated in North America and Europe and freshwaters recover from anthropogenic acidification, Ca concentrations rapidly decline towards a state where they again become balanced with carbonate alkalinity. Ca concentrations may even decline below pre-acidification concentrations due to a depletion of base cations stores in the catchment soils of acid-sensitive regions, or because of other stressors including timber harvesting. Since Ca concentrations are generally low in many freshwaters, in some regions critically low, further Ca concentration declines as freshwaters fully recover from acidification will likely have widespread consequences for biota and ecosystem processes.Emerging threats and persistent conservation challenges for freshwater biodiversity
Andrea J. Reid et al., 2018. Biological Reviews.
(11) Declining calcium
Most aquatic environmental threats are related to the excess of a limiting nutrient (i.e. eutrophication) or a chemical contaminant that exceeds safe concentrations. By contrast, relatively few anthropogenic stressors are related to diminishing supplies of limiting nutrients. One example of a recently identified threat is the slow but widespread decline in calcium (Ca) concentrations in low-carbonate systems across
eastern North America.., Europe…and likely elsewhere. Ca is an essential nutrient for all forms of life, but the ecological ramifications of this new threat are still not fully understood Although Ca-rich dust may play a role (Hedin et al., 1994), the principal source of Ca to freshwaters is the slowweathering of parent bedrock that supplies the Ca pool within catchment soils, which is then potentially available for export to lakes and rivers. Growing evidence shows that human activities have disrupted the Ca cycle of many softwater lakes, reducing the supply of Ca and lowering aqueous Ca concentrations below the demands of some aquatic organisms through two major processes (Jeziorski & Smol, 2017). First, acid rain accelerated the leaching of Ca into lakes, and so, for a period of time lake-water Ca concentrations were likely elevated . In areas with geology characterised by high Ca concentrations (e.g. limestone bedrock), Ca continued to be easily leached into waterways. However, in many low-Ca regions, such as those underlain byPrecambrian granitic bedrock, Ca supplies were eventually depleted as the maintenance of suitable concentrations is mainly dependent on slow weathering processes. Second, as large amounts of Ca are bound up in timber, forestry practices can act as a net export of some of the catchment’s Ca reserves, exacerbating watershed Ca loss… Identifying the ecological effects of long-term Ca declines has, thus far, primarily focused on the Cladocera, often a dominant and keystone group of lake invertebrates. Early analyses revealed that some large-bodied cladocerans (e.g. some Daphnia spp.) have relatively high Ca requirements …with some populations unable to persist should, for example, ambient Ca concentrations fall below 1.5 mg/l. Given that monitoring programs were already recording lower Ca concentrations in many softwater lake regions, concerns were raised that this environmental threat may be affecting lake food webs. A common thread is that Ca declines have been slow and gradual, requiring either palaeolimnological … or long-term monitoring data on the order of decades to identify the problem (Molot & Dillon, 2008). For these reasons, Jeziorski et al. (2008) used analyses of fossil Cladocera to show that, indeed, major shifts in invertebrate assemblages could be linked to declining Ca levels. They found that many softwater lakes were already showing signs of Ca depletion with concomitant changes in cladoceran assemblages. Furthermore, the palaeolimnological data indicated that the recent declines in Ca concentrations recorded in the lake-monitoring programs were not simply a trend of Ca levels rebounding to pre-acidification levels (as one would have expected higher concentrations of Ca in lakes during the early periods of lake acidification), but that current Ca levels were now lower than pre-acidification concentrations. Palaeolimnologists reached this conclusion because Ca-sensitive Daphnia taxa were often common in the pre-acidification fossil record, indicating that Ca levels were sufficiently high prior to acidification. Subsequent studies confirmed this overall trend in a spectrum of softwater lake ecosystems, which may also impact other groups of freshwater biota that have high Ca requirements (reviewed in Jeziorski & Smol, 2017), such as crayfish (Hadley et al., 2015). Although the study of declining Ca was initially focused on taxa impacted by reduced Ca availability (e.g. large-bodied Daphnia), subsequent research has begun to centre on organisms that may benefit from this new threat. For example, given that large Daphnia are efficient filter feeders, their demise may be linked to recent algal blooms, due to reduced top-down effects (Korosi et al., 2012). In addition, Jeziorski et al. (2015) documented the widespread replacement of Daphnia with Holopedium glacialis, a jelly-clad competitor with low Ca requirements. Although both are filter-feeding planktivores, Holopedium have lower nutrient content than Daphnia and high concentrations of the jelly-clad Holopedium can disrupt water filtration equipment. The ensuing ‘jellification of lakes’ is a new problem which potentially can cascade through the food web. The solution to the threat of Ca declines is not a simple one given the large number of affected lakes and their typically remote locations. Further reductions in acidic precipitation is potentially a long-term solution, although one with significant economic repercussions. On a smaller scale, some local attempts have been initiated to replenish Ca in watersheds by, for example, ‘fertilising’ them with Ca-rich wood ash (e.g. Haliburton, Ontario, Canada). The efficacy of these pilot projects has not yet been evaluated.
Birds
Adverse Effects of Acid Rain on the Distribution of the Wood Thrush Hylocichla mustelina in North America.
Hames, R. S.; Rosenberg, K. V.; Lowe, J. D.; Barker, S. E.; Dhondt, A. A.Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 11235−11240. “…We show a strong, highly significant, negative effect of acid rain on the predicted probability of breeding by this species, and interactions with elevation, low pH soils, and habitat fragmentation that worsen these negative effects. Our results suggest an important role for acid rain in recent declines of some birds breeding in the eastern United States, particularly in high elevation zones with low pH soils, and show the need to consider other large-scale influences, in addition to habitat fragmentation, when addressing bird population declines.”
Terrestrial liming benefits birds in an acidified forest in the Northeast.
Pabian, S. E.; Brittingham, M. C. Ecol. Appl. 2007, 17, 2184− 2194.”..We found a significant positive relationship between soil calcium and bird abundance, indicating that soil calcium may affect bird abundance. Liming increased soil calcium and pH and led to increased snail and bird abundances. After liming, bird abundance was positively related to snail abundance. No significant changes occurred in Ovenbird territory size or eggshell thickness. Our results suggest that acid deposition could be responsible for reduced bird abundance, and that liming is a potential mitigation technique.”Forest Herbs
Acidification and Forest Understory Plant Communities in the Adirondack Mountains
Summary Report 2017. Prepared for: New York State Energy Research and Development Authority Albany, New York Prepared by: E&S Environmental Chemistry, Inc. in cooperation with U.S. Geological Survey and State University of New York College of Environmental Sciences and Forestry ” This project focuses on relationships between understory vegetation and soil properties as influenced by acidic deposition in Adirondack Mountain forests. Acidic deposition has reduced the acid-buffering capacity of soils by depleting soil reserves of the important nutrients, calcium and magnesium, at many locations. The research reported here determined the associations between acidic deposition and soil base cation supply and understory plant community responses that reflect aspects of biodiversity. During an earlier field study of Acer saccharum (sugar maple) trees and soil chemistry, 50 study plots within 20 small Adirondack watersheds were sampled and evaluated for soil acid-base chemistry and A. saccharum growth, canopy condition, and regeneration. For this follow-up study, we characterized how understory plant community composition changed at these same sampling locations across deposition and soil acidification gradients using ordination analysis‹nonmetric multidimensional scaling (NMDS). Trees growing on soils with poor acid-base chemistry that received relatively high levels of atmospheric sulfur and nitrogen deposition exhibited minimal to no A. saccharum seedling regeneration, relatively poor canopy condition, and short- to long-term growth declines compared with study plots having better soil condition and lower levels of acidic deposition. Understory species richness was positively related to both exchangeable calcium concentrations and base saturation in both the Oa and upper B soil horizons. Several plant species were strongly and positively correlated with Axis 1 of the NMDS, suggesting positive responses to increases in pH, base saturation, and availability of plant base cation nutrients. Other plant species were negatively correlated with Axis 1, and richness decreased with acidic deposition and soil acidification. Results of this research suggested that plant understory richness in Adirondack hardwood forests was controlled significantly by acidic deposition and soil acidbase chemistry. Both bivariate and multivariate analyses clearly illustrated an association between the base status of the Oa and upper B soil horizons and plant understory richness and species composition.”Plant richness and composition in hardwood forest understories vary along an acidic deposition and soil-chemical gradient in the northeastern United States
Michael R. Zarfos et al., 2019 Plant and Soil volume 438, pages461–477. ” A century of atmospheric deposition of sulfur and nitrogen has acidified soils and undermined the health and recruitment of foundational tree species in the northeastern US. However, effects of acidic deposition on the forest understory plant communities of this region are poorly documented. We investigated how forest understory plant species composition and richness varied across gradients of acidic deposition and soil acidity in the Adirondack Mountains of New York State…The relationship we found between
understory plant communities and a soil-chemical gradient, suggests that soil acidification can reduce diversity and alter the composition of these communities in northern hardwood forests exposed to acidic deposition.Habitat may limit herb migration at the northern edge of the Appalachian deciduous forest
N. Hill and D. Garbury 2011 in Botany Vol 89 (Cited above with Abstract)Trees
Critical Loads and Exceedances of Acid Deposition and Associated Forest Growth in the Northern Hardwood and BorealConiferous Forests in Québec, Canada
Rock Ouimet et al., 2001. Water, Air and Soil Pollution: Focus volume 1, pages119–134 “…We found a significant negative correlation between forest growth rates and critical soil acidification exceedance for both the northern hardwood and the boreal conifer sites.Specifically, plots with critical load exceedances were found to have a growth reduction of about 30% during the 1974–1982 andthe 1972–1990 measurement (plots with no soil acidificationexceedance served as a control). While this correlation is notnecessarily causal, it is nevertheless consistent with the expectation that increased losses of soil base cations on account of increased soil acidification should and could lead to deteriorating forest health conditions.”Long-term decline of sugar maple following forest harvest, Hubbard Brook Experimental Forest, New Hampshire
Natalie L. Cleavitt eta;., 2017 Canadian Journal of Forest Research “Forest harvesting can impact site quality by removing essential nutrients, exacerbating the effects of historic base cation losses associated with acid deposition. We studied the 30-year trajectory of forest recovery from clearcutting (whole-tree harvest (WTH)) in a forest originally dominated by sugar maple (Acer saccharum Marsh.). At both the watershed scale (21.9 ha) and the “detailed” plot scale (1 m2), a dramatic decline of sugar maple was observed, along with maintenance of American beech (Fagus grandifolia Ehrh.) and an increase in birch, mainly yellow birch (Betula alleghaniensis Britt.). Many of the “detailed” plots where sugar maple failed to recruit became unoccupied rather than being “won” by another species. The decline of sugar maple was most severe in the upper elevation zones of the watershed, where low base status (especially Ca) of the soils was a likely driver. The results support previous studies indicating that regeneration by sugar maple is severely compromised on base cation depleted soils. Lower survival of seedlings for sugar maple emphasized the importance of maintaining advance regeneration to favor desired species such as sugar maple. Foresters should consider that sites with low base saturation and exchangeable Ca are likely to exhibit regeneration failure for sugar maple in the long term, even those with initial dominance by this species.”Some Watershed-level effects of forest management
Cation storage and availability along a Nothofagus forest development sequence in New Zealand
R B Allen et al., 1997. Canadian Journal of Forest Research “Soil cations and pH were determined in relation to the development of even-aged (10, 25, 120, and >150-year-oldstands) mountain beech (Nothofagus solandri var. cliffortioides (Hook.f.) Poole) forest after catastrophic canopy disturbance.Live stem biomass varied from 1 to 273 Mg/ha between the seedling (10 years) and pole (120 years) stages, respectively, but was less in the mature stage (>150 years; 245 Mg/ha). Coarse woody debris mass declined monotonically from 168.7 Mg/hain the seedling stage to 23.7 Mg/ha in the mature stage. Total cation (Ca, Mg, and K) storage in wood (live stem biomass pluscoarse woody debris) was highest in the pole stage and least in the sapling stage (25 years) because sapling stands had lowstem biomass and only intermediate levels of woody debris. This matched high soil cation availability in the sapling stage andlow availability in the pole stage. Between these stages soil pH declined and inorganic monomeric aluminium increased. The seedling and mature stages often had intermediate levels of soil-available cations and pH. This study does not support the hypothesis that sequestering of cations in aggrading biomass necessarily results in a monotonic decline in soil cation availability as forests develop; instead mountain beech exhibits a bimodal pattern for soil cations. The reciprocal oscillation of nutrients between living wood, deadwood, and the soil contributes in a major way to these patterns.”Watmough, S. A., Aherne, J. & Dillon, P. J. (2003). Potential impact of forest harvesting on lake chemistry in south-central Ontario at current levels of acid deposition. Canadian Journal of Fisheries and Aquatic Sciences 60, 1095–1103. “The critical loads method based on the Stable Water Chemistry Model (SSWC) allowed us to determine the potential impact of forest harvesting on ~1300 lakes in south central Ontario. The critical acid load is currently exceeded by gross sulphate deposition in only 9% of the lakes, in the absence of forest harvesting. However, in the following three potential harvest scenarios, wood alone (trunks without bark), trunks alone and whole trees, the percentages increase to 23%, 56% and 72% respectively. This increase in exceedance of the critical load is explained by much lower concentrations of base cations in the lakes, due to their removal during harvesting. Thus, only 0.3% of the lakes have Ca 2+ concentrations <–1 in the absence of harvesting, whereas 52% of the lakes have such concentrations when the whole trees are harvested. Forest harvesting therefore has a significant potential impact on lake chemistry, which becomes more apparent as soil exchangeable base cation stores decline and acid inputs to lakes are no longer buffered. ”
Base cation reservoirs in soil control the buffering capacity of lakes in forested catchments.
Houle, D., R. Ouimet, S. Couture, and C. Gagnon. 2006 Can. J. Fish. Aquat. Sci. 63: 471-474. PDF. ABSTRACT “The acidification of forest soils and surface waters and their relatively poor recovery record following reductions in atmospheric sulfur emissions is a major ongoing environmental problem, particularly in northeastern North America. The slow recovery of surface water is widely hypothesized to result from depletion of reservoirs of base cations in soil. This is concordant with the theory that the acid-neutralizing capacity (ANC) of lakes is likely proportional to the size of the exchangeable base cation reservoirs present in surrounding watershed soils. However, data describing these linkages are still nonexistent in the literature. Here we show that lake ANC is highly predictable ( r 2 = 0.75) based on the size of the exchangeable Ca 2+ reservoir in soil in 21 catchments representative of soil and lake conditions encountered in northeastern North America. This finding indirectly supports the hypothesis that the poor recovery of surface water from acidification is governed by the size of base cation reservoirs present in catchment soils. The size of the base cation reservoir in soil is thus a strong indicator of the acid–base status of both soils and surface waters.” and from the Conclusions (links inserted) “:… The relationship described here strongly supports this hypothesis and demonstrates that base cation reservoir size, particularly Ca2+, is a strong indicator of the acid–base status of both soils and surface waters. When these considerations are added to the mounting evidence that basic cation reservoir size is also a predictor of tree growth and health (Shortle et al. 1997; Duchesne et al. 2002), our data strongly support the argument that soil base cation reservoirs should be designated as key variables in the management of terrestrial and aquatic ecosystems in forested areas.” - A Simple Geospatial Nutrient Budget Model for Assessing Forest Harvest Sustainability across Nova Scotia, Canada
